Biomedical engineering is the application of engineering principles and techniques to the medical field. This field seeks to close the gap between engineering and medicine: It combines the design and problem solving skills of engineering with medical and biological sciences to improve healthcare diagnosis, monitoring and therapy.[1]
Biomedical engineering has only recently emerged as its own discipline, compared to many other engineering fields; such an evolution is common as a new field transitions from being an interdisciplinary specialization among already-established fields, to being considered a field in itself. Much of the work in biomedical engineering consists of research and development, spanning a broad array of subfields (see below). Prominent biomedical engineering applications include the development of biocompatible prostheses, various diagnostic and therapeutic medical devices ranging from clinical equipment to micro-implants, common imaging equipment such as MRIs and EEGs, biotechnologies such as regenerative tissue growth, and pharmaceutical drugs and biopharmaceuticals.
Contents |
Subdisciplines within biomedical engineering
Biomedical engineering is a highly interdisciplinary field, influenced by (and overlapping with) various other engineering and medical fields. This often happens with newer disciplines, as they gradually emerge in their own right after evolving from special applications of extant disciplines. Due to this diversity, it is typical for a biomedical engineer to focus on a particular subfield or group of related subfields. There are many different taxonomic breakdowns within BME, as well as varying views about how best to organize them and manage any internal overlap; the main U.S. organization devoted to BME divides the major specialty areas as follows:[2]- Biomechatronics
- Bioinstrumentation
- Biomaterials
- Biomechanics
- Bionics
- Cellular, Tissue, and Genetic Engineering
- Clinical Engineering
- Medical Imaging
- Orthopaedic Bioengineering
- Rehabilitation engineering
- Systems Physiology
- Bionanotechnology
- Neural Engineering
- Chemical engineering - often associated with biochemical, cellular, molecular and tissue engineering, biomaterials, and biotransport.
- Electrical engineering - often associated with bioelectrical and neural engineering, bioinstrumentation, biomedical imaging, and medical devices. This also tends to encompass Optics and Optical engineering - biomedical optics, imaging and related medical devices.
- Mechanical engineering - often associated with biomechanics, biotransport, medical devices, and modeling of biological systems, like soft tissue mechanics.
Biotechnology and pharmaceuticals
Biotechnology (see also relatedly bioengineering) can be a somewhat ambiguous term, sometimes loosely used interchangeably with BME in general; however, it more typically denotes specific products which use "biological systems, living organisms, or derivatives thereof." [2] Even some complex "medical devices" (see below) can reasonably be deemed "biotechnology" depending on the degree to which such elements are central to their principle of operation. Biologics/Biopharmaceuticals (e.g., vaccines, stored blood product), genetic engineering, and various agricultural applications are some major classes of biotechnology.Pharmaceuticals are related to biotechnology in two indirect ways: 1) certain major types (e.g. biologics) fall under both categories, and 2) together they essentially comprise the "non-medical-device" set of BME applications. (The "Device - Bio/Chemical" spectrum is an imperfect dichotomy, but one regulators often use, at least as a starting point.)
Tissue engineering
Main article: Tissue engineering
Tissue engineering is a major segment of Biotechnology.One of the goals of tissue engineering is to create artificial organs (via biological material) for patients that need organ transplants. Biomedical engineers are currently researching methods of creating such organs. Researchers have grown solid jawbones[3] and tracheas from human stem cells towards this end. Several artificial urinary bladders actually have been grown in laboratories and transplanted successfully into human patients.[4] Bioartificial organs, which use both synthetic and biological components, are also a focus area in research, such as with hepatic assist devices that use liver cells within an artificial bioreactor construct.[5]

Micromass cultures of C3H-10T1/2 cells at varied oxygen tensions stained with Alcian blue.
Genetic Engineering
Genetic engineering, recombinant DNA technology, genetic modification/manipulation (GM) and gene splicing are terms that apply to the direct manipulation of an organism's genes.[1] Genetic engineering is different from traditional breeding, where the organism's genes are manipulated indirectly. Genetic engineering uses the techniques of molecular cloning and transformation to alter the structure and characteristics of genes directly. Genetic engineering techniques have found success in numerous applications. Some examples are in improving crop technology (not a medical application per se; see BioSystems Engineering), the manufacture of synthetic human insulin through the use of modified bacteria, the manufacture of erythropoietin in hamster ovary cells, and the production of new types of experimental mice such as the oncomouse (cancer mouse) for research.Neural Engineering
Neural engineering (also known as Neuroengineering) is a discipline that uses engineering techniques to understand, repair, replace, or enhance neural systems. Neural engineers are uniquely qualified to solve design problems at the interface of living neural tissue and non-living constructs.Pharmaceutical engineering
Pharmaceutical Engineering is sometimes regarded as a branch of biomedical engineering, and sometimes a branch of chemical engineering; in practice, it is very much a hybrid sub-discipline (as many BME fields are). Aside from those pharmaceutical products directly incorporating biological agents or materials, even developing chemical drugs is considered to require substantial BME knowledge due to the physiological interactions inherent to such products' usage.Medical devices
This is an extremely broad category -- essentially covering all health care products that do not achieve their intended results through predominantly chemical (e.g., pharmaceuticals) or biological (e.g., vaccines) means, and do not involve metabolism.A medical device is intended for use in:
- the diagnosis of disease or other conditions, or
- in the cure, mitigation, treatment, or prevention of disease,
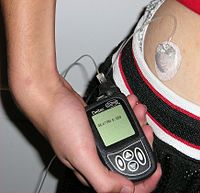
A pump for continuous subcutaneous insulin infusion, an example of a biomedical engineering application of electrical engineering to medical equipment.
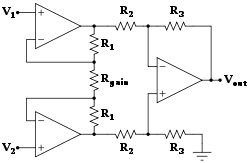
Biomedical instrumentation amplifier schematic used in monitoring low voltage biological signals, an example of a biomedical engineering application of electronic engineering to electrophysiology.
Medical devices are regulated and classified (in the US) as follows (see also Regulation):
- Class I devices present minimal potential for harm to the user and are often simpler in design than Class II or Class III devices. Devices in this category include tongue depressors, bedpans, elastic bandages, examination gloves, and hand-held surgical instruments and other similar types of common equipment.
- Class II devices are subject to special controls in addition to the general controls of Class I devices. Special controls may include special labeling requirements, mandatory performance standards, and postmarket surveillance. Devices in this class are typically non-invasive and include x-ray machines, PACS, powered wheelchairs, infusion pumps, and surgical drapes.
- Class III devices generally require premarket approval (PMA) or premarket notification (510k), a scientific review to ensure the device's safety and effectiveness, in addition to the general controls of Class I. Examples include replacement heart valves, hip and knee joint implants, silicone gel-filled breast implants, implanted cerebellar stimulators, implantable pacemaker pulse generators and endosseous (intra-bone) implants.
Medical imaging
Main article: Medical imaging
Medical/biomedical imaging is a major segment of medical devices. This area deals with enabling clinicians to directly or indirectly "view" things not visible in plain sight (such as due to their size, and/or location). This can involve utilizing ultrasound, magnetism, UV, other radiology, and other means.
An MRI scan of a human head, an example of a biomedical engineering application of electrical engineering to diagnostic imaging. Click here to view an animated sequence of slices.
- Fluoroscopy
- Magnetic resonance imaging (MRI)
- Nuclear medicine
- Positron emission tomography (PET) PET scansPET-CT scans
- Projection radiography such as X-rays and CT scans
- Tomography
- Ultrasound
- Optical microscopy
- Electron microscopy
Implants
An implant is a kind of medical device made to replace and act as a missing biological structure (as compared with a transplant, which indicates transplanted biomedical tissue). The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone or apatite depending on what is the most functional. In some cases implants contain electronics e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents.
Artificial limbs: The right arm is an example of a prosthesis, and the left arm is an example of myoelectric control.

A prosthetic eye, an example of a biomedical engineering application of mechanical engineering and biocompatible materials to ophthalmology.
Clinical engineering
Main article: Clinical engineering
Clinical engineering is the branch of biomedical engineering dealing with the actual implementation of medical equipment and technologies in hospitals or other clinical settings. Major roles of clinical engineers include training and supervising biomedical equipment technicians (BMETs), selecting technological products/services and logistically managing their implementation, working with governmental regulators on inspections/audits, and serving as technological consultants for other hospital staff (e.g. physicians, administrators, I.T., etc.). Clinical engineers also advise and collaborate with medical device producers regarding prospective design improvements based on clinical experiences, as well as monitor the progression of the state-of-the-art so as to redirect procurement patterns accordingly.Their inherent focus on practical implementation of technology has tended to keep them oriented more towards incremental-level redesigns and reconfigurations, as opposed to revolutionary research & development or ideas that would be many years from clinical adoption; however, there is a growing effort to expand this time-horizon over which clinical engineers can influence the trajectory of biomedical innovation. In their various roles, they form a "bridge" between the primary designers and the end-users, by combining the perspectives of being both 1) close to the point-of-use, while 2) trained in product and process engineering. Clinical Engineering departments will sometimes hire not just biomedical engineers, but also industrial/systems engineers to help address operations research/optimization, human factors, cost analysis, etc. Also see safety engineering for a discussion of the procedures used to design safe systems.
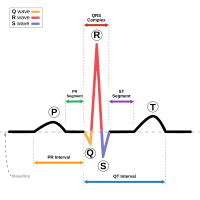
Schematic representation of a normal ECG trace showing sinus rhythm; an example of widely-used clinical medical equipment (operates by applying electronic engineering to electrophysiology and medical diagnosis.
Regulatory issues
Regulatory issues are of particular concern to a biomedical engineer; it is among the most heavily-regulated fields of engineering, and practicing biomedical engineers must routinely consult and cooperate with regulatory law attorneys and other experts. The Food and Drug Administration (FDA) is the principal healthcare regulatory authority in the United States, having jurisdiction over medical devices, drugs, biologics, and combination products. The paramount objectives driving policy decisions by the FDA are safety and efficacy of healthcare products.[citation needed]In addition, because biomedical engineers often develop devices and technologies for "consumer" use, such as physical therapy devices (which are also "medical" devices), these may also be governed in some respects by the Consumer Product Safety Commission. The greatest hurdles tend to be 510K "clearance" (typically for Class 2 devices) or pre-market "approval" (typically for drugs and class 3 devices).

Implants, such as artificial hip joints, are generally extensively regulated due to the invasive nature of such devices.
The different regulatory arrangements sometimes result in particular technologies being developed first for either the U.S. or in Europe depending on the more favorable form of regulation. While nations often strive for substantive harmony to facilitate cross-national distribution, philosophical differences about the optimal extent of regulation can be a hindrance; more restrictive regulations seem appealing on an intuitive level, but critics decry the tradeoff cost in terms of slowing access to life-saving developments.
Training and certification
Education
Biomedical engineers require considerable knowledge of both engineering and biology, and typically have a Master's (M.S., M.S.E., or M.Eng.) or a Doctoral (Ph.D.) degree in BME or another branch of engineering with considerable potential for BME overlap. As interest in BME is increasing, many engineering colleges now have a Biomedical Engineering Department or Program, with offerings ranging from the undergraduate (B.S. or B.S.E.) to the doctoral levels. As noted above, biomedical engineering has only recently been emerging as its own discipline rather than a cross-disciplinary hybrid specialization of other disciplines; now, BME programs of study at all levels are becoming more widespread, including the Bachelor of Science in Biomedical Engineering which actually includes so much biological science content that many students use it as a "pre-med" major in preparation for medical school. The number of biomedical engineers is expected to rise as both a cause and effect of improvements in medical technology.[6]In the U.S., an increasing number of undergraduate programs are also becoming recognized by ABET as accredited bioengineering/biomedical engineering programs. Over 65 programs are currently accredited by ABET.[7][8]
As with many degrees, the reputation and ranking of a program may factor into the desirability of a degree holder for either employment or graduate admission. The reputation of many undergraduate degrees are also linked to the institution's graduate or research programs, which have some tangible factors for rating, such as research funding and volume, publications and citations. With BME specifically, the ranking of a university's hospital and medical school can also be a significant factor in the perceived prestige of its BME department/program.
Graduate education is a particularly important aspect in BME. While many engineering fields (such as mechanical or electrical engineering) do not need graduate-level training to obtain an entry-level job in their field, the majority of BME positions do prefer or even require them.[9] Since most BME-related professions involve scientific research, such as in pharmaceutical and medical device development, graduate education is almost a requirement (as undergraduate degrees typically do not involve sufficient research training and experience). This can be either a Masters or Doctoral level degree; while in certain specialties a Ph.D. is notably more common than in others, it is hardly ever the majority (except in academia). In fact, the perceived need for some kind of graduate credential is so strong that some undergraduate BME programs will actively discourage students from majoring in BME without an expressed intention to also obtain a masters degree or apply to medical school afterwards.
Graduate programs in BME, like in other scientific fields, are highly varied, and particular programs may emphasize certain aspects within the field. They may also feature extensive collaborative efforts with programs in other fields (such as the University's Medical School or other engineering divisions), owing again to the interdisciplinary nature of BME. M.S. and Ph.D. programs will typically require applicants to have an undergraduate degree in BME, or another engineering discipline (plus certain life science coursework), or life science (plus certain engineering coursework).
Education in BME also varies greatly around the world. By virtue of its extensive biotechnology sector, its numerous major universities, and relatively few internal barriers, the U.S. has progressed a great deal in its development of BME education and training opportunities. Europe, which also has a large biotechnology sector and an impressive education system, has encountered trouble in creating uniform standards as the European community attempts to supplant some of the national jurisdictional barriers that still exist. Recently, initiatives such as BIOMEDEA have sprung up to develop BME-related education and professional standards.[10] Other countries, such as Australia, are recognizing and moving to correct deficiencies in their BME education.[11] Also, as high technology endeavors are usually marks of developed nations, some areas of the world are prone to slower development in education, including in BME.
Licensure/Certification
See also: Professional engineer
Engineering licensure in the US is largely optional, and rarely specified by branch/discipline. As with other learned professions, each state has certain (fairly similar) requirements for becoming licensed as a registered professional engineer (PE), but in practice such a license is not required to practice in the majority of situations (duee to an exception known as the private industry exemption, which effectively applies to the vast majority of American engineers). This is notably not the case in many other countries, where a license is as legally necessary to practice engineering as it is for law or medicine.Biomedical engineering is regulated in some countries, such as Australia, but registration is typically only recommended and not required.[12]
In the UK, mechanical engineers working in the areas of Medical Engineering, Bioengineering or Biomedical engineering can gain Chartered Engineer status through the Institution of Mechanical Engineers. The Institution also runs the Engineering in Medicine and Health Division.[13]
The Fundamentals of Engineering exam - the first (and more general) of two licensure examinations for most U.S. jurisdictions—does now cover biology (although technically not BME). For the second exam, called Part 2 or the Professional Engineering exam, candidates may select a particular engineering discipline's content to be tested on; there is currently not an option for BME with this, meaning that any biomedical engineers seeking a license must prepare to take this examination in another category (which does not affect the actual license, since most jurisdictions do not recognize discipline specialties anyway). However, the Biomedical Engineering Society (BMES) is, as of 2009, exploring the possibility of seeking to implement a BME-specific version of this exam to facilitate biomedical engineers' pursuing licensure.
Beyond governmental registration, certain private-sector professional/industrial organizations also offer certifications with varying degrees of prominence. One such example is the Certified Clinical Engineer (CCE) certification for Clinical engineers.
Founding figures
- Leslie Geddes (deceased)- Professor Emeritus at Purdue University, electrical engineer, inventor and educator of over 2000 biomedical engineers, received a National Medal of Technology in 2006 from President George Bush[14] for his more than 50 years of contributions that have spawned innovations ranging from burn treatments to miniature defibrillators, ligament repair to tiny blood pressure monitors for premature infants, as well as a new method for performing cardiopulmonary resuscitation (CPR).
- Y. C. Fung - professor emeritus at the University of California, San Diego, considered by many to be the founder of modern Biomechanics[15]
- Robert Langer - Institute Professor at MIT, runs the largest BME laboratory in the world, pioneer in drug delivery and tissue engineering[16]
- Herbert Lissner (deceased) - Professor of Engineering Mechanics at Wayne State University. Initiated studies on blunt head trauma and injury thresholds beginning in 1939 in collaboration with Dr. E.S. Gurdjian, a neurosurgeon at Wayne State's School of Medicine. Individual for whom the American Society of Mechanical Engineering's top award in Biomedical Engineering, the Herbert R. Lissner Medal, is named.
- Nicholas A. Peppas - Chaired Professor in Engineering, University of Texas at Austin, pioneer in drug delivery, biomaterials, hydrogels and nanobiotechnology.
- Otto Schmitt (deceased) - biophysicist with significant contributions to BME, working with biomimetics
- Ascher Shapiro (deceased) - Institute Professor at MIT, contributed to the development of the BME field, medical devices (e.g. intra-aortic balloons)
- John G. Webster - Professor Emeritus at the University of Wisconsin–Madison, a pioneer in the field of instrumentation amplifiers for the recording of electrophysiological signals
- Robert Plonsey - Professor Emeritus at Duke University, pioneer of electrophysiology[17]
- U. A. Whitaker (deceased) - provider of The Whitaker Foundation, which supported research and education in BME by providing over $700 million to various universities, helping to create 30 BME programs and helping finance the construction of 13 buildings[18]
- Frederick Thurstone (deceased) - Professor Emeritus at Duke University, pioneer of diagnostic ultrasound[19]
- Alfred E. Mann - Physicist, entrepreneur and philanthropist. A pioneer in the field of Biomedical Engineering.[20]
- Forrest Bird - aviator and pioneer in the invention of mechanical ventilators
- Willem Johan Kolff (deceased) - pioneer of hemodialysis as well as in the field of artificial organs
- Medical device
Notes
- ^ Biomedical engineer prospects
- ^ [1], About BMES - Specialty Areas within BME
- ^ "Jaw bone created from stem cells". BBC News. October 10, 2009. http://news.bbc.co.uk/2/hi/health/8290138.stm. Retrieved 11 October 2009.
- ^ "Doctors grow organs from patients' own cells". CNN. April 3, 2006. http://www.cnn.com/2006/HEALTH/conditions/04/03/engineered.organs/index.html.
- ^ Trial begins for first artificial liver device using human cells, University of Chicago, February 25, 1999
- ^ U.S. Bureau of Labor Statistics - Profile for Engineers
- ^ Accredited Biomedical Engineering Programs
- ^ ABET List of Accredited Engineering Programs
- ^ U.S. Bureau of Labor Statistics - Job Outlook for Engineers
- ^ BIOMEDEA
- ^ Biomedical Engineering Curriculum: A Comparison Between the USA, Europe and Australia
- ^ National Engineering Registration Board - Areas Of Practice - NPER Areas
- ^ http://www.imeche.org/industries/medical/
- ^ YouTube - Leslie Geddes - 2006 National Medal of Technology
- ^ YC “Bert” Fung: The Father of Modern Biomechanics (pdf)
- ^ Colleagues honor Langer for 30 years of innovation, MIT News Office
- ^ Faculty - Duke BME
- ^ The Whitaker Foundation
- ^ Biomedical Engineering Professor Emeritus Fredrick L. Thurstone Dies
- ^ http://www.aemf.org/The Alfred E. Mann Foundation for Scientific Research (AMF)
Further reading
- Bronzino, Joseph D. (April 2006). The Biomedical Engineering Handbook, Third Edition. [CRC Press]. ISBN 9780849321245. http://crcpress.com/product/isbn/9780849321245.
- Villafane, Carlos, CBET. (June 2009). Biomed: From the Student's Perspective, First Edition. [Techniciansfriend.com]. ISBN 9781615396634. http://www.biomedtechnicians.com.
External links
| At Wikiversity you can learn more and teach others about Biomedical engineering at: |